Osteomyelitis, primarily caused by Staphylococcus aureus infection, is a prevalent condition. While over 100,000 fungal species are described, only 150 are pathogenic to humans. Opportunistic infections, often when host defenses are compromised or through invasive gateways like dental extractions or traumatic skin discontinuities, pose challenges in timely diagnosis and treatment.
This case study involves a healthy 13-year-old boy without immune incompetency, presenting with pain, swelling, and intermittent fever over 15 days in his left ankle and leg. Clinical evaluation revealed sub-acute osteomyelitis of the distal tibia with septic arthritis. Despite negative bacterial culture, positive findings in potassium hydroxide mount (hyphae and pseudohyphae) and Candida growth in fungal culture indicated a rare fungal infection. Managing fungal osteomyelitis is intricate due to its infrequent occurrence in bones.
This case contributes insights into diagnosing and managing a rare pediatric osteomyelitis variant. Treatment guidelines vary based on the identified organism and treatment duration. Surgical debridement for fungal osteomyelitis or septic arthritis involves removing sinus tracts, assessing for squamous cell carcinoma, bony and soft-tissue debridement, and placement of antibiotic or antifungal beads. The spectrum of osteomyelitis ranges from Staphylococcus aureus to tumors, necessitating a comprehensive investigation. Despite their rarity, fungal infections should be considered differentials. Early diagnosis, surgical intervention, and appropriate antifungal treatment based on the identified species enhance clinical outcomes.
Introduction: Pediatric Fungal Osteomyelitis
Osteomyelitis, an infection affecting bone and bone marrow, is predominantly instigated by Staphylococcus aureus [1]. It manifests as acute or chronic inflammation, impacting bone and its associated structures due to pyogenic organisms, including bacteria, viruses, fungi, and mycobacterial species [2]. Staphylococcus aureus is responsible for 30% to 60% of human cases, with staphylococci collectively contributing to approximately 75% of occurrences [3][4]. The primary modes of transmission involve a hematogenous route, often following a fracture or surgical procedure.
While the catalog of fungal species exceeds 100,000, only 150 are pathogenic to humans [5]. Aspergillus and Candida species are common fungal pathogens in septic arthritis and osteomyelitis [6]. Opportunistic infections, gaining entry due to compromised host defense, nutritional deficiencies, or invasive gateways like traumatic skin discontinuities, are prevalent causes [7]. The challenge arises from symptoms and radiological findings that closely resemble other etiologies, leading to significant delays in initiating treatment. Aspergillus infection frequently targets the vertebral bone, with fungal osteomyelitis being a rare occurrence in the pediatric population and often prone to misdiagnosis [8]. Due to the limited literature, we present a case report detailing fungal osteomyelitis in a pediatric patient.
Case Presentation [1]
1. Patient Presentation
- A 13-year-old healthy boy with no history of immune incompetency.
- Presented with complaints of pain, swelling over the left ankle and leg, and intermittent fever for 15 days.
2. Hematological Findings
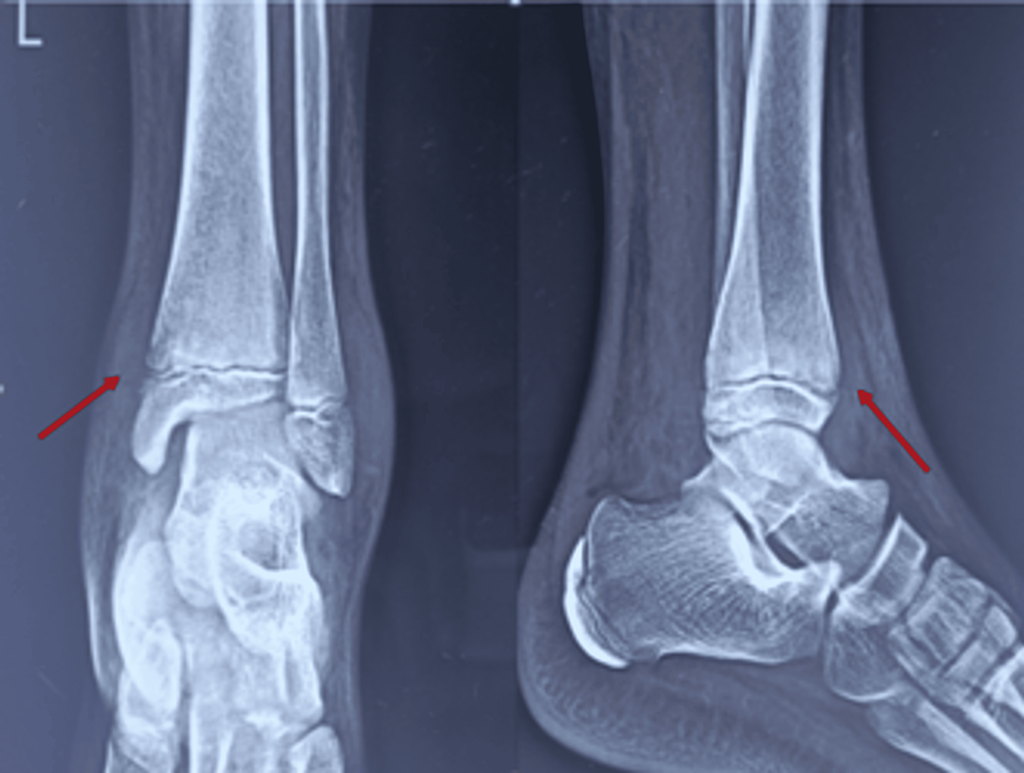
Figure 1: Plane radiograph showing soft tissue outline with swelling and regional osteopenia over the anteromedial aspect of the distal tibia with irregularity of the physeal margin (arrow)
- Routine investigations revealed leukocytosis, elevated CRP, and ESR.
- In-plane radiographs displayed soft tissue outline, anteromedial swelling of the distal tibia, physical margin irregularity, and osteopenia without periosteal reaction (Figure 1).
3. MRI Findings
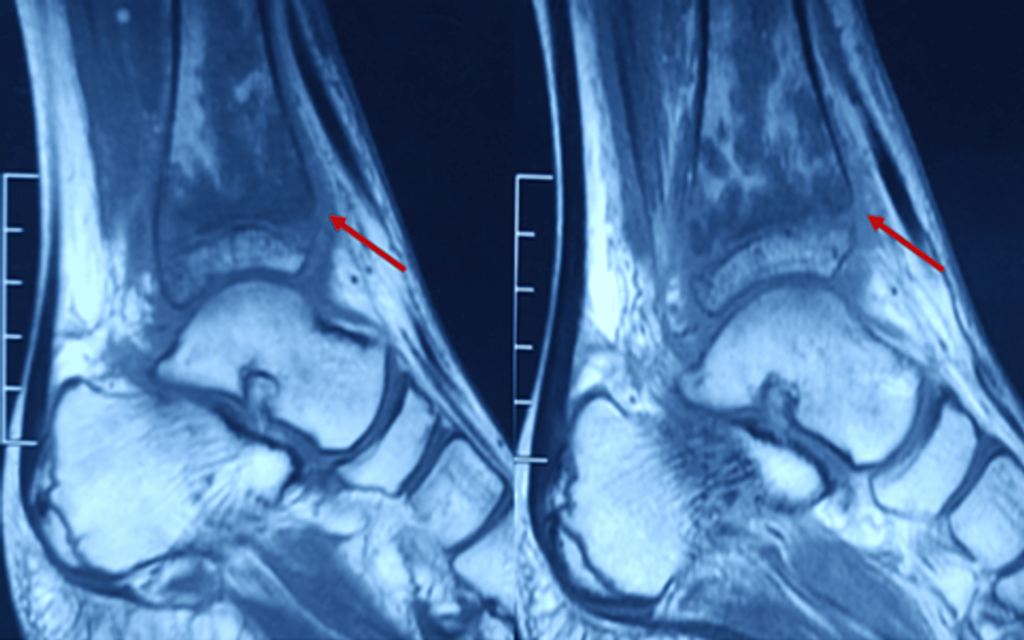
Figure 2: Sagittal MRI section of the distal tibia showing osteomyelitis changes (arrow)
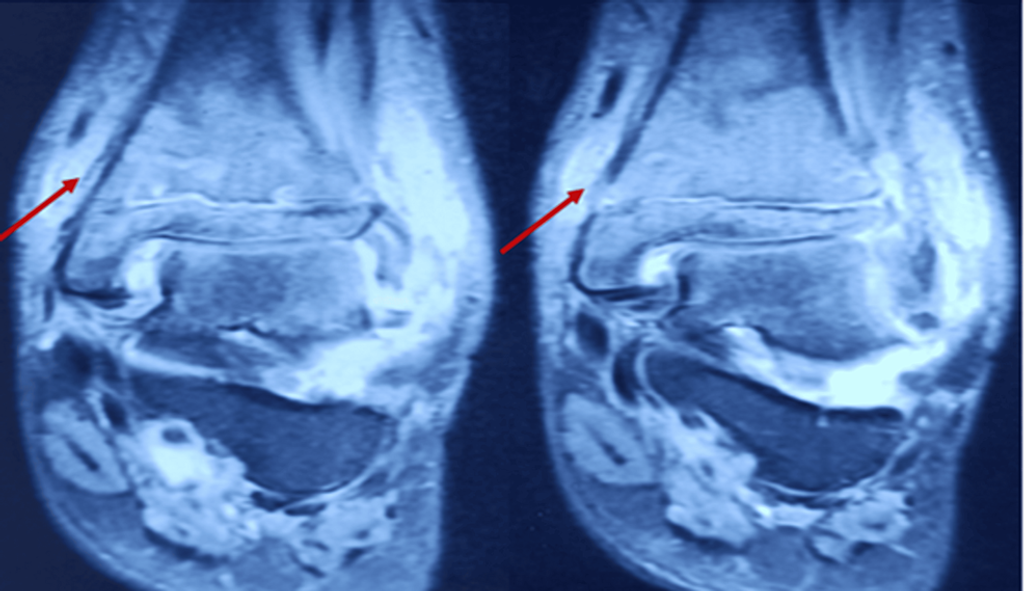
Figure 3: T2 coronal MRI section showing osteomyelitis changes in the distal tibia (arrow)
- MRI indicated nodular synovial thickening with effusion in the ankle joint.
- Serpiginous intramedullary bone infarcts were observed (Figures 2-3).
4. Diagnosis and Surgical Procedure
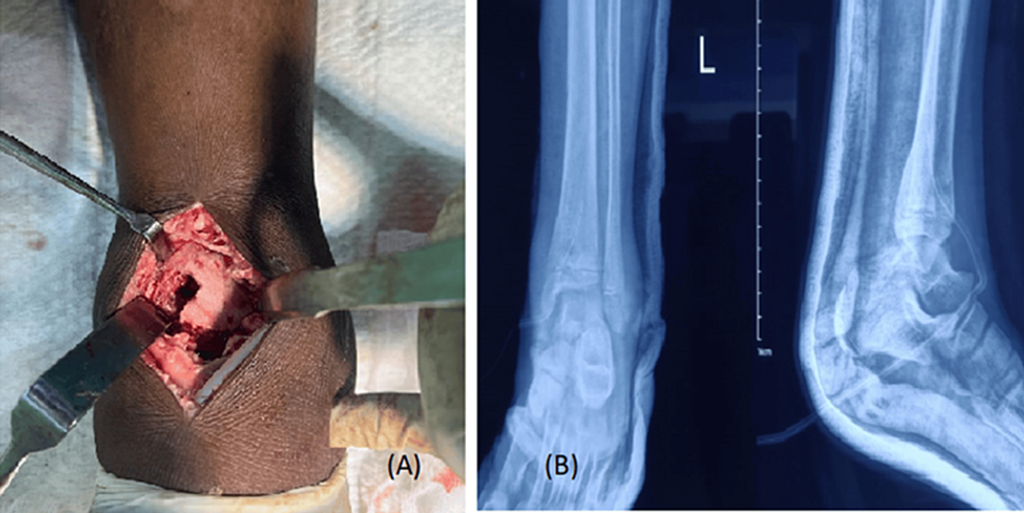
Figure 4: (A) Distal tibial cortical window and (B) postoperative radiograph
- Diagnosed with osteomyelitis of the distal tibia and septic arthritis.
- The preoperative period involved no antibiotic administration.
- Supine is positioned on the operation table with a vertical anterior incision between both malleoli.
- Superficial dissection, creating a deep plane, identifying and retracting the neurovascular bundle, and incising the joint capsule.
- Non-purulent collection was observed during distal tibial cortical window creation (Figure 4A).
5. Laboratory Investigations
- Aspirates and soft tissue samples were sent for various tests: Gram stain, aerobic and anaerobic culture sensitivity, nucleic acid amplification test, acid-fast bacteria stain, KOH mount, and histopathological examination.
6. Postoperative Care
- Wound closure in layers, drain insertion, and postoperative dressing on days 2 and 5.
- Sutures were removed 14 days after the initial surgery.
- A postoperative radiograph was taken (Figure 4B).
7. Postoperative Medication and Recovery
- Prophylactic initiation of ceftriaxone and vancomycin.
- Decrease in total leukocyte counts, ESR, and CRP trends.
- Bacterial culture showed no growth, but KOH mount and fungal culture were positive for Candida, sensitive to fluconazole.
- Intravenous fluconazole was administered for 28 days, resulting in complete swelling subsidence in 18 days and no residual ankle stiffness.
Discussion
Managing fungal infections poses challenges due to their infrequent occurrence in bones. Fungal osteomyelitis, especially in the pediatric population, is a rare entity in the literature, and the current case is explored for its diagnosis and management as a unique variant of osteomyelitis. The clinical presentation of bacterial and fungal osteomyelitis exhibits similarities [9], and fungal osteomyelitis is an unusual manifestation often coexisting with septic arthritis [10].
Treatment guidelines vary depending on the identified organism and the treatment duration. Debridement of fungal osteomyelitis or septic arthritis involves:
- Removing sinus tracts,
- Evaluating for squamous cell carcinoma,
- Conducting bony and soft-tissue debridement and
- Placing antibiotic or antifungal beads.
In cases of fungal septic arthritis, comprehensive surgical irrigation and debridement are performed. However, surgical interventions may not be obligatory for managing certain fungi, such as Cryptococcus, when the disease burden is mild [6]. Fluconazole, known for inhibiting the demethylation of C-14 sterols, accumulating abnormal methyl sterols, enhancing cellular permeability, and exerting inhibitory effects on fungal cells [8], is a preferred treatment option. This approach aligns with Pan et al.’s case report, where fluconazole demonstrated favorable outcomes in treating Candida osteomyelitis in pediatric patients [12].
Conclusion
The spectrum of osteomyelitis encompasses entities from Staphylococcus aureus organisms to tumors, underscoring the importance of thorough investigation across the entire spectrum of the disease. Despite its rarity, fungal infection should be considered a potential differential diagnosis. Timely diagnosis, surgical debridement, and appropriate antifungal treatment tailored to the specific fungal species significantly improve clinical outcomes and results.
References
- Afaque S, Agrawal U, Shankhwar D K, et al. A Rare Case of Fungal Osteomyelitis of the Distal Tibia in a Pediatric Patient. 2024 February, 21 Cureus 16(2): e54648. DOI: doi:10.7759/cureus.54648
- Hofstee MI, Muthukrishnan G, Atkins GJ, et al.: Current concepts of osteomyelitis: from pathologic mechanisms to advanced research methods. Am J Pathol. 2020, 190:1151-63. 10.1016/j.ajpath.2020.02.007.
- Schmitt SK: Musculoskeletal infections: meeting the challenge. Infect Dis Clin North Am. 2017, 31: ix-x. 10.1016/j.idc.2017.03.001
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr: Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015, 28:603-61. 10.1128/CMR.00134-14
- Walter G, Kemmerer M, Kappler C, Hoffmann R: Treatment algorithms for chronic osteomyelitis. Dtsch Arztebl Int. 2012, 109:257-64. 10.3238/arztebl.2012.0257.
- Bongomin F, Gago S, Oladele RO, Denning DW: Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel). 2017, 3:57. 10.3390/jof3040057.
- Bariteau JT, Waryasz GR, McDonnell M, Fischer SA, Hayda RA, Born CT: Fungal osteomyelitis and septic arthritis. J Am Acad Orthop Surg. 2014, 22:390-401. 10.5435/JAAOS-22-06-390.
- Bhansali A, Bhadada S, Sharma A, et al.: Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J. 2004, 80:670-4. 10.1136/pgmj.2003.016030.
- Asperges E, Albi G, Truffelli F, et al.: Fungal osteomyelitis: a systematic review of reported cases. Microorganisms. 2023, 11:1828. 10.3390/microorganisms11071828.
- Urs AB, Singh H, Mohanty S, Sharma P: Fungal osteomyelitis of maxillofacial bones: a rare presentation. J Oral Maxillofac Pathol. 2016, 20:546. 10.4103/0973-029X.190966 Fungal Osteomyelitis. (2022). Accessed: 2023 September 18: https://radiopaedia.org/articles/fungal-osteomyelitis.
- Fleming L, Ng A, Paden M, Stone P, Kruse D: Fungal osteomyelitis of calcaneus due to Candida albicans: a case report. J Foot Ankle Surg. 2012, 51:212-4. 10.1053/j.jfas.2011.07.007
- Pan N, Herzog R, Blanco JS, et al.: Candida albicans osteomyelitis in an infant: a case report and literature review. J Pediatr Orthop B. 2013, 22:491-7. 10.1097/BPB.0b013e3283613313
About Docquity
If you need more confidence and insights to boost careers in healthcare, expanding the network to other healthcare professionals to practice peer-to-peer learning might be the answer. One way to do it is by joining a social platform for healthcare professionals, such as Docquity.
Docquity is an AI-based state-of-the-art private & secure continual learning network of verified doctors, bringing you real-time knowledge from thousands of doctors worldwide. Today, Docquity has over 400,000 doctors spread across six countries in Asia. Meet experts and trusted peers across Asia where you can safely discuss clinical cases, get up-to-date insights from webinars and research journals, and earn CME/CPD credits through certified courses from Docquity Academy. All with the ease of a mobile app available on Android & iOS platforms!







