Colorectal cancer poses a significant global health challenge, with nearly 1.9 million new diagnoses and 935,000 deaths annually. Early detection is crucial for successful treatment, and population-based screening programs are pivotal in identifying cases at an earlier, more treatable stage.
A recent study by the Netherlands Cancer Institute has unveiled promising results for a new stool test, the Multitarget-FIT (mtFIT), suggesting it may outperform the current standard, the fecal immunochemical test (FIT). This breakthrough holds the potential further to reduce colorectal cancer cases and associated mortality rates.
The Current Screening Landscape
Colorectal cancer screening programs, such as those in the Netherlands, have been instrumental in diagnosing the disease at earlier stages. However, the current FIT test, which measures the presence of hemoglobin, has limitations. It performs well but falls short in detecting tumors before they become invasive, particularly at the stage of larger premalignant polyps.
The Multitarget-FIT Test
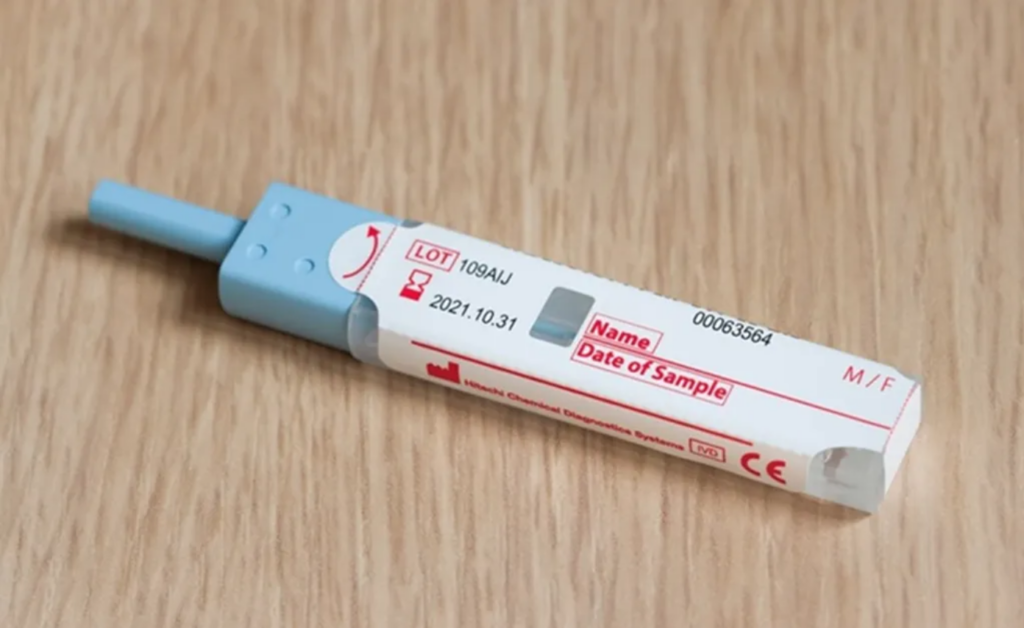
The mtFIT test, developed by researchers at the Netherlands Cancer Institute, Amsterdam UMC, and Erasmus MC, measures hemoglobin and two additional proteins. In a prospective study involving over 13,000 participants in the Dutch national population-based screening program, the mtFIT demonstrated its efficacy in detecting cancer precursors more effectively than the current FIT test.
Key Findings
The study revealed that the mtFIT test yielded more positive results than the FIT test, leading to increased colonoscopies. However, crucially, the mtFIT test identified abnormalities in 299 individuals, compared to 159 with the FIT test. Importantly, the majority of these abnormalities were high-risk precursors of colon cancer. According to the principal investigator Gerrit Meijer, “The new test detects larger polyps without a significant increase in ‘false-positive’ results and thus unnecessary colonoscopies.”
Impact on Colorectal Cancer Cases and Mortality
The promising results suggest that the mtFIT test could lead to a substantial reduction in both colorectal cancer cases and mortality. In the Dutch screening program, the mtFIT test, with its higher sensitivity, could potentially result in 21% fewer cases of colorectal cancer and 18% fewer mortalities. The figures may be slightly lower in countries with lower FIT cutoff values, but significant reductions in both instances and mortality are anticipated.
Cost-Effectiveness and Implementation
One notable aspect of the mtFIT test is its potential cost-effectiveness. While the exact numbers vary based on the screening program’s FIT cutoff values, it is projected that the mtFIT test could be a cost-effective solution for colorectal cancer screening.
Implementing the mtFIT in existing FIT-based screening programs is expected to be relatively straightforward, given the similarity in screening logistics between the two tests. This ease of integration makes transitioning to the mtFIT test a feasible option for enhancing current screening efforts.
Future Steps
Gerrit Meijer emphasizes that the mtFIT test does not replace the current population screening test. The critical next step involves producing the test on an industrial scale according to European diagnostic test guidelines. To facilitate this, the researchers have established a Dutch Diagnostic Company dedicated to ensuring that the mtFIT test benefits colorectal cancer screening participants in the Netherlands and globally.
Conclusion
The discovery of the Multitarget-FIT test’s efficacy in detecting colorectal cancer precursors marks a significant milestone in the quest for more accurate and efficient screening methods. With the potential to reduce the number of colorectal cancer cases and associated mortalities, the mtFIT test holds promise for improving population-based colorectal cancer screening worldwide. Establishing a diagnostic company signals a commitment to making this groundbreaking test widely accessible, offering hope for a future where colorectal cancer is detected and treated at an even earlier, more curable stage.
Medical Disclaimer: The information, including but not limited to text, graphics, images, and other material contained on this website, is for informational purposes only. No material on this site is intended to be a substitute for professional medical advice, diagnosis, or treatment.
Reference
Pieter H A Wisse, Willemijn de Klaver, Francine van Wifferen, Frejanne G van Maaren-Meijer, Huub E van Ingen, Lana Meiqari, et al., The multitarget faecal immunochemical test for improving stool-based colorectal cancer screening programmes: a Dutch population-based, paired-design, intervention study.The Lancet Oncology. February 09, 2024. DOI: https://doi.org/10.1016/S1470-2045(23)00651-4
About Docquity
If you need more confidence and insights to boost careers in healthcare, expanding the network to other healthcare professionals to practice peer-to-peer learning might be the answer. One way to do it is by joining a social platform for healthcare professionals, such as Docquity.
Docquity is an AI-based state-of-the-art private & secure continual learning network of verified doctors, bringing you real-time knowledge from thousands of doctors worldwide. Today, Docquity has over 400,000 doctors spread across six countries in Asia. Meet experts and trusted peers across Asia where you can safely discuss clinical cases, get up-to-date insights from webinars and research journals, and earn CME/CPD credits through certified courses from Docquity Academy. All with the ease of a mobile app available on Android & iOS platforms!