Sneddon syndrome, also called livedo reticularis with cerebrovascular accidents, is a rare yet persistent condition impacting blood vessels in both the skin and the brain. It is characterized by a net-like pattern on the skin known as livedo reticularis, resulting from blood vessel constriction. Apart from skin changes, Sneddon syndrome often presents with recurrent neurological events like strokes or transient ischemic attacks, varying in severity and leading to potential complications. During admission to the stroke unit, a 28-year-old female displayed bilateral livedo reticularis on her soles and the dorsal sides of her hands. Diagnosis involves a thorough assessment comprising medical history, physical examination, routine laboratory tests, and additional diagnostic procedures.
Introduction: Sneddon Syndrome
Sneddon syndrome (SS) is a rare, chronic vascular disorder affecting predominantly young adults aged 20 to 42, with a higher incidence among women [1]. It presents two main features: livedo racemosa (LR) and neurological symptoms [2]. LR is a dermatological condition characterized by persistent, branching skin discoloration [3]. Neurological symptoms can vary widely, from migraines to severe manifestations such as strokes or seizures, sometimes preceding LR onset [4,5]. Though its exact cause is unknown, genetic factors, autoimmune responses, and coagulation disorders are implicated [1]. SS is often associated with retinal changes, cardiac valve involvement, and systolic labile hypertension, with potential risk factors including estrogen-containing contraceptives, pregnancy, smoking, diabetes, atherosclerosis, and hyperlipidemia [5]. Diagnosis entails a comprehensive assessment involving a medical history review, physical examination, blood tests, imaging studies, and occasionally skin biopsy.
Case Presentation
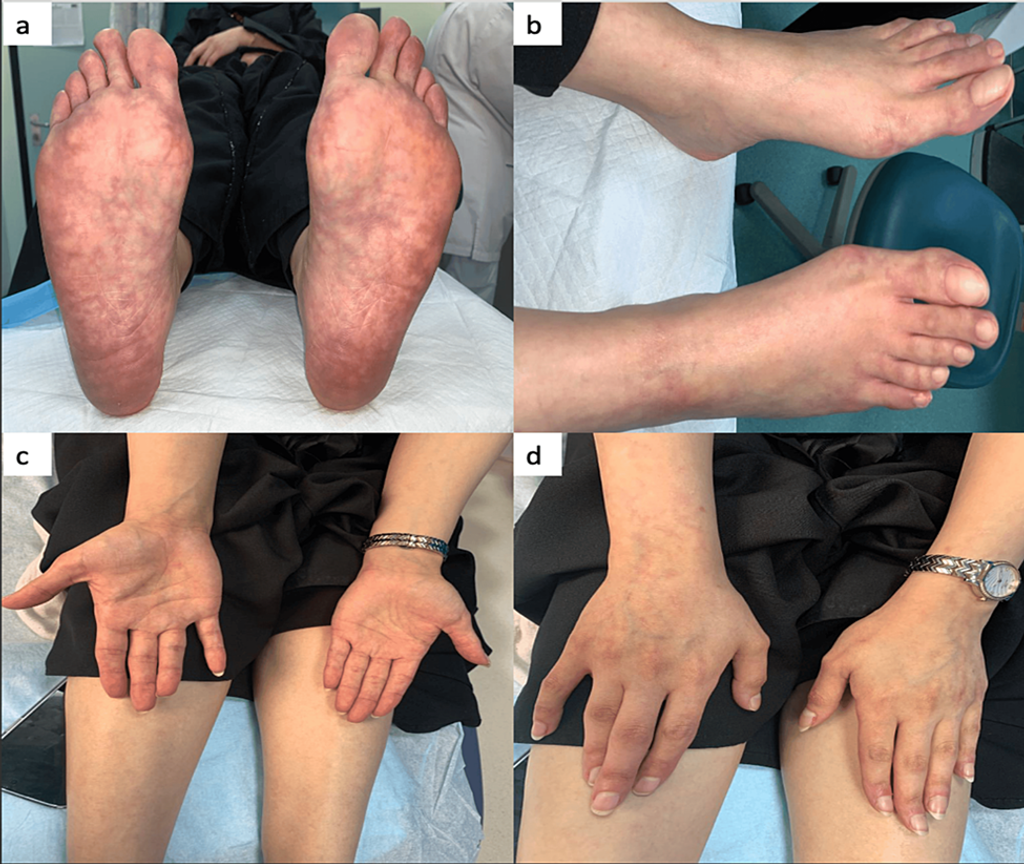
Figure 1: Multiple erythematous-to-violaceous irregular reticular patterns with incomplete circular patches (livedo racemosa) on both the plantar and dorsal aspects of her feet and the palmer and dorsal aspects of both hands.
- Presentation
- A 28-year-old female experienced sudden onset symptoms:
- Weakness in the right arm and hand
- Facial asymmetry
- Slurred speech
- A 28-year-old female experienced sudden onset symptoms:
- Examination Findings
- Multiple erythematous-to-violaceous irregular reticular patterns:
- On both plantar and dorsal aspects of the feet
- On the palmar and dorsal aspects of both hands (Figure 1).
- Incomplete circular patches observed
- Normal pulmonary and cardiac auscultation
- Blood pressure: 117/70 mmHg
- Multiple erythematous-to-violaceous irregular reticular patterns:
- Neurological Assessment
- Difficulties with naming and reading
- Intact comprehension, repetition, and writing abilities
- Slightly reduced pinprick sensation in right V2 and V3 compared to the left side
- Patient Background
- History of ischemic stroke seven months ago.
- The stroke affected the left middle cerebral artery, resulting in right-sided hemiplegia.
- Concurrent conditions: Raynaud’s phenomenon and mild protein S deficiency.
- Current medications
- Aspirin (81 mg) and atorvastatin (20 mg) for ischemic stroke.
- Sildenafil (25 mg) and amlodipine (5 mg) for Raynaud’s phenomenon.
- Differential Diagnosis
- Protein S deficiency
- Antiphospholipid syndrome
- Vasculitis
- Sjögren’s syndrome (SS)
- Oral contraceptive pill use
- Livedo reticularis
- Laboratory Results:
- Complete blood count, coagulation profile, and renal and liver functions within normal ranges.
- Negative results for autoimmune antibodies (including ANA, ANCA, Smith antibody, SS antibody, dsDNA ab, JO-1 antibody, cardiolipin antibody, β2-glycoprotein I antibody, myeloperoxidase, PR-3, and C3 and C4).
- Negative hemophilia screening (JAK2 mutation, FVL mutation, FII mutation).
- Negative infectious disease screening (syphilis, VDRL CSF, Brucella CSF, hepatitis B and C, HIV).
- Vasculitis Screening
- Protein S level is low, but protein C is normal.
- Negative direct immunofluorescence studies for IgG, IgM, IgA, and C3.
- Fibrinogen showed scattered positivity without specific indications of vasculitis or thrombotic vasculopathy.
- Stroke Workup
- Unremarkable results for transthoracic echocardiography, transesophageal echocardiography, renal artery Doppler, and 48-hour Holter monitoring.
- Computed tomography (CT) scan showed no evidence of acute, well-established territorial infarction or intracranial hemorrhage.
- CT angiography showed no filling defects.
- Brain magnetic resonance imaging (MRI) revealed an acute ischemic infarct in the territory of the left middle cerebral artery, affecting the insula and the frontoparietal lobe and extending to the pre- and post-central gyri. Additionally, chronic small-vessel disease characteristics were noted (Figure 2).
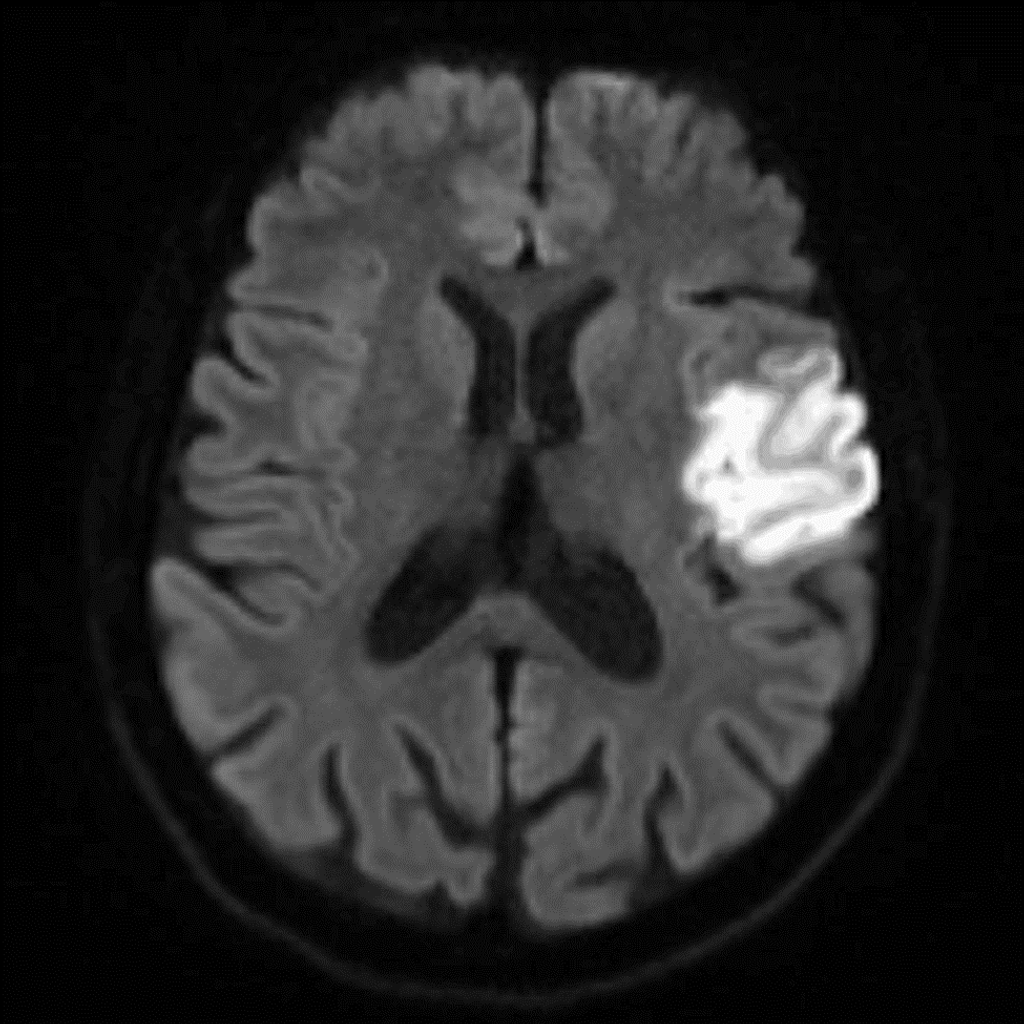
Figure 2: Brain magnetic resonance imaging showing an acute ischemic infarct in the left middle cerebral artery territory, affecting the insula and the frontoparietal lobe and extending to the pre-and post-central gyri.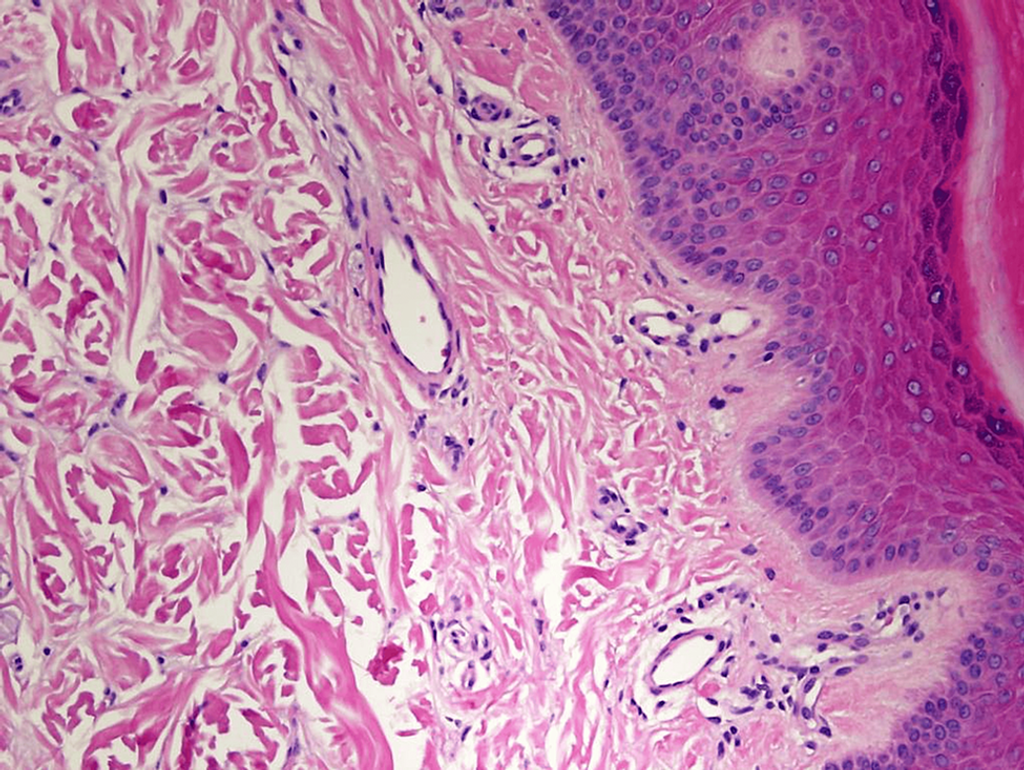
Figure 3: High-power photomicrograph of the epidermis and papillary dermis showing no significant pathological changes (hematoxylin and eosin stain, original magnification 400×).
- Punch Biopsy Results
- Microscopic examination of a biopsy from the patient’s right foot showed normal and patent small dermal blood vessels (Figure 3).
- Focal subcutaneous fat necrosis indicated ischemic changes (Figure 4).
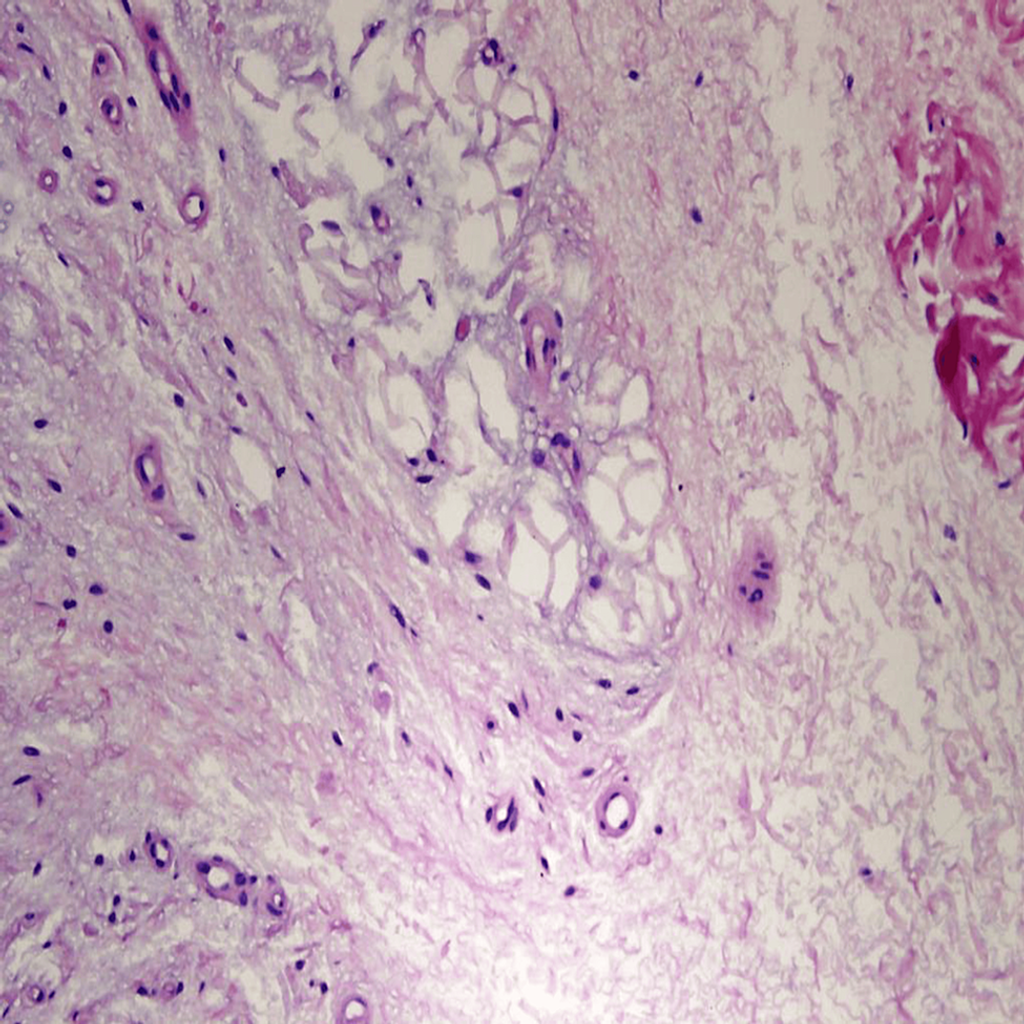
Figure 4: High-power photomicrograph of a skin punch biopsy showing focal fat necrosis (hematoxylin and eosin stain, original magnification 400×). No active inflammation or vasculitis was seen.
- Medical History
- After the biopsy, the patient experienced a miscarriage at 11-12 weeks of pregnancy.
- The patient also had another episode of limb weakness and numbness a few months later.
- Current Medical Management:
- The patient is under the care of the neurology clinic with no identified source of stroke at present.
- Medications include nifedipine, simvastatin, aspirin (81 mg), and angiotensin-converting enzyme inhibitors.
- Cyclophosphamide was added for eight months for a patient with seronegative SS and cognitive and behavioral decline.
- Treatment Outcome:
- Following treatment with cyclophosphamide, the patient improved subjective and objective memory and emotional status.
- Treatment Recommendation:
- Based on the observed improvement, cyclophosphamide is suggested as a potential first-line treatment for SS patients with cognitive impairment.
Discussion on Sneddon Syndrome
SS is a rare vasculopathy disorder characterized by cerebrovascular issues with livedo reticularis (LR). Its annual incidence is approximately 4 per 1 million, primarily affecting young women [1]. SS can be categorized into three types: primary (without identified causative factors), autoimmune (associated with antiphospholipid antibodies, systemic lupus erythematosus, or lupus-like disease), and thrombophilic [6]. While the disorder typically occurs sporadically, a few familial cases have been documented [7]. SS presents with various systemic and dermatological symptoms. LR, defined as an irregular, net-like erythematous-to-violaceous pattern on the skin, may precede stroke onset by years [3]. While LR commonly affects the trunk or buttocks in nearly all patients [1], our case showcases a rare presentation with LR on acral regions. In some instances, LR may emerge after neurological symptoms [5]. Another cutaneous manifestation of SS is Raynaud’s phenomenon, which affects the hands and feet.
Stroke represents a significant diagnostic feature of SS, typically resulting from ischemia [1]. Hemiparesis, sensory disturbances, aphasia, and visual field defects rank among the most prevalent clinical indications. Cognitive impairment and depression may affect around 77% of SS patients [8]. While intracerebral, subarachnoid, or intraventricular hemorrhages are uncommon in SS, hypertension is present in 15-65% of cases. Ischemic heart disease has also been documented. Furthermore, approximately 65% of SS patients exhibit reduced creatinine clearance [9]. Spontaneous abortion has been linked to SS as well. A prior investigation revealed that out of 38 SS patients, 32 (84%) were female. Among these, three experienced recurrent miscarriages, seven had unexplained miscarriages, and three encountered fertility issues [10].
The management of SS aims to manage symptoms and mitigate the risk of complications. While a definitive cure remains elusive, a multidisciplinary strategy involving medication to prevent clotting, regulate blood pressure, and decrease inflammation is typically employed [5]. Addressing potential risk factors through weight management, abstaining from smoking, and adopting regular exercise routines is advisable. Although coping with SS can present challenges, adequate medical attention and support can yield positive outcomes. Continuous research endeavors are underway to enhance comprehension of this condition and uncover novel treatment modalities, providing optimism for individuals impacted by SS.
Conclusions on Sneddon Syndrome
Sneddon Syndrome (SS) is a rare neurocutaneous vasculopathy known to impair quality of life significantly. Livedo reticularis (LR) and recurrent neurovascular events are defining features of this condition. Although the precise etiology of SS remains uncertain, ongoing research indicates the possible involvement of autoimmune or genetic factors. Managing SS effectively necessitates a multidisciplinary approach encompassing medical interventions, lifestyle modifications, and consistent monitoring. Timely diagnosis and intervention can enable fulfilling lives while mitigating the impact of this disorder.
References
- Wu S, Xu Z, Liang H: Sneddon’s syndrome: a comprehensive review of the literature. Orphanet J Rare Dis. 2014, 9:215. 10.1186/s13023-014-0215-4
- Sneddon IB: Cerebro-vascular lesions and livedo reticularis. Br J Dermatol. 1965, 77:180-5. 10.1111/j.1365-2133. 1965.tb14628.x
- Pincelli MS, Echavarria AM, Criado PR, Marques GF, Morita TC, Valente NY, de Carvalho JF: Livedo racemosa: clinical, laboratory, and histopathological findings in 33 patients. Int J Low Extrem Wounds. 2021, 20:22-8. 10.1177/1534734619896938
- Cleaver J, Teo M, Renowden S, Miller K, Gunawardena H, Clatworthy P: Sneddon syndrome: a case report exploring the current challenges faced with diagnosis and management. Case Rep Neurol. 2019, 11:357-68. 10.1159/000503955
- Samanta D, Cobb S, Arya K: Sneddon syndrome: a comprehensive overview. J Stroke Cerebrovasc Dis. 2019, 28:2098-108. 10.1016/j.jstrokecerebrovasdis.2019.05.013
- Schellong SM, Weissenborn K, Niedermeyer J, Wollenhaupt J, Sosada M, Ehrenheim C, Lubach D: Classification of Sneddon’s syndrome. Vasa. 1997, 26:215-21.
- Szmyrka-Kaczmarek M, Daikeler T, Benz D, Koetter I: Familial inflammatory Sneddon’s syndrome-case report and review of the literature. Clin Rheumatol. 2005, 24:79-82. 10.1007/s10067-004-0981-9
- Adair JC, Digre KB, Swanda RM, Hartshorne MF, Lee RR, Constantino TM, Knoefel JE: Sneddon’s syndrome: a cause of cognitive decline in young adults. Neuropsychiatry Neuropsychol Behav Neurol. 2001, 14:197-204.
- Zelger B, Sepp N, Stockhammer G, et al.: Sneddon’s syndrome. A long-term follow-up of 21 patients. Arch Dermatol. 1993, 129:437-47. 10.1001/archderm.129.4.437
- Dikova Ch, Kolarov G, Baleva M, Nikolov K: [Spontaneous abortions in Sneddon’s syndrome]. Akush Ginekol (Sofiia). 2000, 39:24-5.
About Docquity
If you need more confidence and insights to boost careers in healthcare, expanding the network to other healthcare professionals to practice peer-to-peer learning might be the answer. One way to do it is by joining a social platform for healthcare professionals, such as Docquity.
Docquity is an AI-based state-of-the-art private & secure continual learning network of verified doctors, bringing you real-time knowledge from thousands of doctors worldwide. Today, Docquity has over 400,000 doctors spread across six countries in Asia. Meet experts and trusted peers across Asia where you can safely discuss clinical cases, get up-to-date insights from webinars and research journals, and earn CME/CPD credits through certified courses from Docquity Academy. All with the ease of a mobile app available on Android & iOS platforms!






