Xenotransplantation, or cross-species transplantation, can address the critical shortage of human tissues for clinical transplantation. This shortage currently prevents most waiting-list patients from receiving the transplants they require. From the 17th to the 20th centuries, blood transfusions from various animal species were performed on patients with diverse pathological conditions. In the 19th century, skin grafts from multiple animals, with frogs being the most popular donor, were conducted.
In the 1920s, Voronoff advocated for the transplantation of slices of chimpanzee testis into aging men experiencing a decline in their “zest for life,” believing that the hormones produced by the testis would rejuvenate his patients. Building on Carrel’s pioneering work on blood vessel anastomosis, numerous attempts at non-human primate organ transplantation were made in the 20th century. In 1963–1964, facing a shortage of human organs and the absence of chronic dialysis, Reemtsma transplanted chimpanzee kidneys into 13 patients. One patient returned to work for almost nine months before suddenly succumbing to what was believed to be an electrolyte disturbance.
The first human heart transplant took place in 1964 when Hardy used a chimpanzee heart, but the patient passed away within 2 hours. In 1966, Starzl conducted the first chimpanzee-to-human liver transplantation, and in 1992, he achieved a patient survival of 70 days following a baboon liver transplant. Advances in genetic engineering and cloning technologies have enabled the development of pigs with various manipulations protecting their tissues from the human immune response, resulting in increased pig graft survival in non-human primate models. The use of genetically modified pigs offers hope for an unlimited supply of organs and cells for those needing transplantation.
Introduction
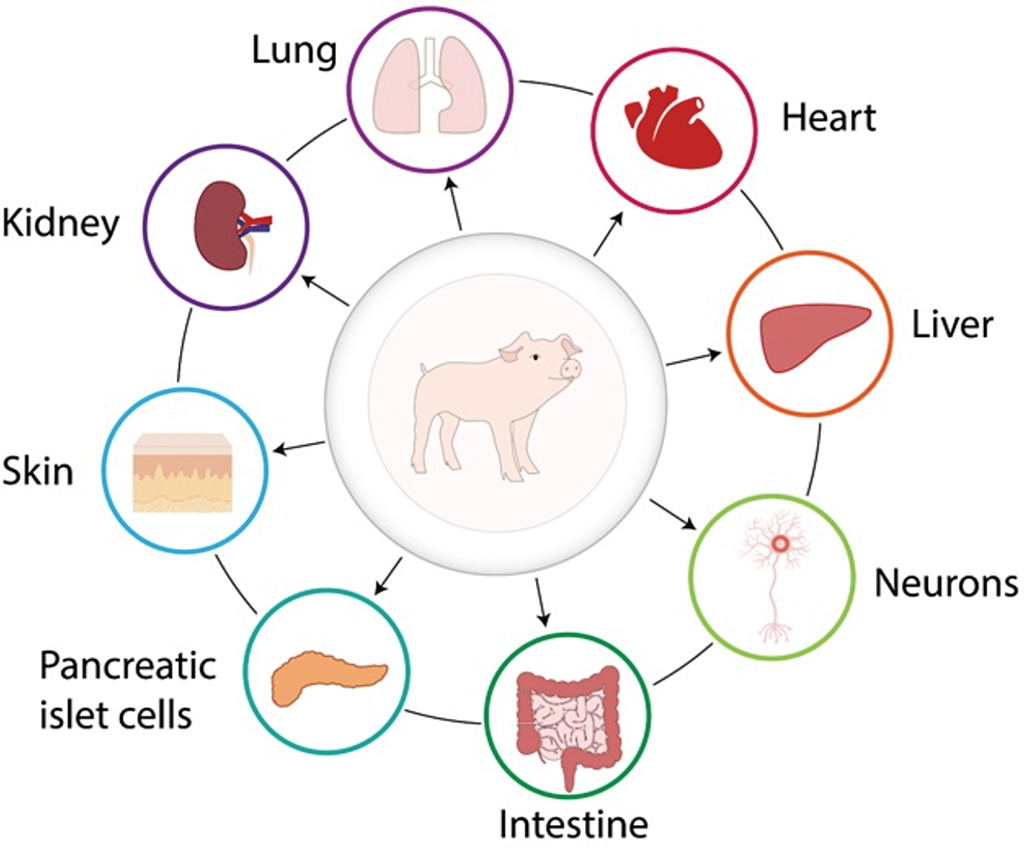
The growing demand for organs, tissues, and cells for clinical transplantation, coupled with a limited increase in the availability of deceased human organs each year, has sparked interest in exploring the use of organs and cells from animal species [1,2]. The concept of cross-species transplantation, known as xenotransplantation, is not a new idea, and there have been numerous clinical attempts over the past 300 years or more [1,3,4]. Overcoming significant barriers to xenotransplantation is an ongoing process, with advancements primarily driven by our ability to genetically modify pigs, making their tissues more resistant to the human immune response.
Xenotransplantation in Mythology
A review of Greek mythology and religious texts, especially those from the Hindu religion, highlights humanity’s long-standing fascination with the prospect of combining physical traits from diverse animal species. Over the centuries, mythological figures like the chimera have symbolized allotransplantation of organs and cells (transplantation within the same species), while the lamassu (Figure 1) has been chosen as the mythological entity representing the International Xenotransplantation Association and its official scientific journal, Xenotransplantation.
 Figure:1 The lamassu. This mythological beast has been adopted for the logo of the International Xenotransplantation Association and its official journal, Xenotransplantation. Image courtesy of IXA.
Figure:1 The lamassu. This mythological beast has been adopted for the logo of the International Xenotransplantation Association and its official journal, Xenotransplantation. Image courtesy of IXA.
Blood Xenotransfusion
If we extend our gaze beyond mythology and legend, we arrive at the 17th century, when Jean Baptiste Denis (Figure 2) initiated the clinical practice of transfusing blood from animals to humans [6]. Unsurprisingly, the outcomes were varied, leading to the prohibition of xenotransfusion in France for a considerable period. In contemporary times, with the growing concern about the transmission of infectious agents through human blood transfusions, a compelling argument can be made for considering animals such as pigs, maintained under optimal “clean” conditions and regularly monitored to prevent the transfer of infectious agents, as potential sources for blood cells and blood products in the future [6]. This approach has indeed been revisited by several research groups recently [7].

Figure 2: Jean-Baptiste Denis (c. 1635–1704). Image courtesy of Musée d’Histoire de la Médecine, Faculté de Médecine de Paris.
Skin Xenotransplantation
During the 19th century, there was a notable rise in the practice of skin grafts between various animal species and humans [8,9]. These grafts were either free skin grafts or pedicle skin grafts. Pedicle grafts posed challenges as they necessitated immobilizing the donor, such as a sheep, to the patient for an extended period. During this time, the graft was believed to establish a blood supply from the recipient. If successful, the graft could then be disconnected from the donor. Despite some reported “successes,” it is highly likely that none of these grafts achieved genuine success. The choice of donor species, including sheep, rabbits, dogs, cats, rats, chickens, and pigeons, possessing hair, feathers, or fur on their skin, did not deter the involved surgeons. However, there was a growing preference for animal species lacking such features. Frogs, sometimes “skinned alive,” were considered ideal donors. Some of these grafts provided temporary protection to cover skin ulcers, allowing for healing beneath the graft over a few days. Nevertheless, none of the grafts probably persisted as permanent solutions.
Corneal Xenotransplantation
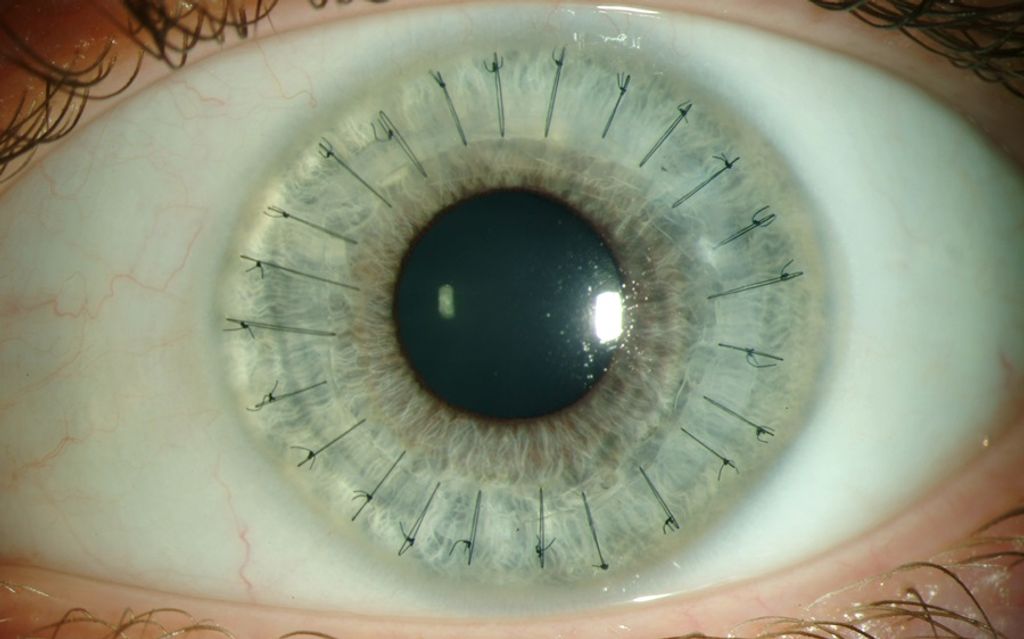 Surprisingly, in 1838, the inaugural corneal xenotransplantation, utilizing tissue from a pig, was conducted on a patient. In contrast, the first corneal allograft (human-to-human) did not occur until over 65 years later, in 1905. The domain of corneal xenotransplantation has undergone recent scrutiny, as outlined in reviews by Hara and Cooper [10,11].
Surprisingly, in 1838, the inaugural corneal xenotransplantation, utilizing tissue from a pig, was conducted on a patient. In contrast, the first corneal allograft (human-to-human) did not occur until over 65 years later, in 1905. The domain of corneal xenotransplantation has undergone recent scrutiny, as outlined in reviews by Hara and Cooper [10,11].
Alexis Carrel and Blood Vessel Anastomosis
 Figure 3: Alexis Carrel (1873–1944), photographed by Huggins.
Figure 3: Alexis Carrel (1873–1944), photographed by Huggins.
Further scientific advancements had to await the 20th century when the French experimental surgeon Alexis Carrel (Figure 3), initially in France and later in North America, pioneered surgical techniques for anastomosing blood vessels. This breakthrough allowed for the successful execution of organ transplantation for the first time, earning him the Nobel Prize in 1912. Carrel also developed an interest in cross-species transplantation, particularly from an experimental standpoint, and in 1907, he penned these prophetic words:
“The ideal method would be to transplant in man organs of animals easy to secure and operate on, such as hogs. However, it is necessary to immunize the organs of the hog against the human serum. The future of transplantation of organs for therapeutic purposes depends on the feasibility of hetero [xeno] transplantation.”
Notably, over 100 years ago, Carrel envisioned what we are currently attempting—genetically modifying pigs to enhance their tissues’ resistance to the human immune response. Carrel was a visionary individual.
“Rejuvenation” by Cell Xenotransplantation

Figure 4: Serge Voronoff (1866–1951). Image from the U.S. Library of Congress (record LC-B2- 6197-1).
Several years later, Serge Voronoff, a Russian immigrant based in Paris, introduced the idea of transplanting cells responsible for producing a deficient hormone in the recipient. He is another example of a forward-thinking scientist who is well ahead of his time. Presently, we are realizing his vision by transplanting human pancreatic islets that generate insulin into patients with severe type 1 diabetes. Given the limited availability of human pancreas annually, there is a growing interest in employing pig islets for this purpose.
However, Voronoff’s primary focus was on reversing aging’s effects on elderly men who had lost their “zest for life.” He conducted many testicular transplants from chimpanzees or baboons to male human recipients [10]. His method involved slicing the animal testicle and inserting the slices into the recipient’s testicle. Despite the popularity of this procedure on both sides of the Atlantic and the execution of several hundred operations, it is unlikely that any had tangible benefits beyond psychological effects. Nevertheless, there were reports of remarkable “rejuvenation,” with men citing significantly increased energy post-operation. Surprisingly, complications were seemingly rare.
The idea of transplanting glandular tissue to produce beneficial hormones for the recipient persisted in the United States, championed by a less scientifically inclined doctor, John Brinkley. His work, predominantly conducted in Kansas and Texas [11], involved using goats as donors due to his belief in their sexual potency, influenced by a local farmer.
Kidney Xenotransplantation

Figure 5: Keith Reemtsma (1925–2000). Image courtesy of the late Keith Reemtsma.
In the 1960s, Keith Reemtsma (Figure 5), then at Tulane University in Louisiana, proposed non-human primate kidneys as a viable treatment for renal failure due to the limited availability of deceased human kidneys and the absence of chronic dialysis. Selecting chimpanzees for their close evolutionary connection to humans, he conducted 13 transplants, each involving both chimpanzee kidneys and transplanting them into recipients [17].
Unfortunately, most transplants fail within 4 to 8 weeks, primarily due to the limited availability of immunosuppressive agents leading to rejection or infectious complications from their excessive use. Despite challenges, one patient survived for nine months, returning to work before unexpectedly collapsing and dying. An autopsy revealed normal chimpanzee kidneys, suggesting no acute or chronic rejection (Figure 6). The suggested cause of death was an acute electrolyte disturbance, possibly linked to the significant diuresis observed in the early post-transplantation period, often exceeding 20 liters in 24 hours, potentially leading to a late electrolyte imbalance.
 Figure 6: Normal macroscopic appearance at autopsy of chimpanzee kidneys (top) that had functioned well for almost nine months in a 23-year-old woman who had undergone renal xenotransplantation in 1963. This operation was one of a small series of kidney xenotransplants performed by Keith Reemtsma and his colleagues at Tulane University in New Orleans. Image courtesy of the late Keith Reemtsma.
Figure 6: Normal macroscopic appearance at autopsy of chimpanzee kidneys (top) that had functioned well for almost nine months in a 23-year-old woman who had undergone renal xenotransplantation in 1963. This operation was one of a small series of kidney xenotransplants performed by Keith Reemtsma and his colleagues at Tulane University in New Orleans. Image courtesy of the late Keith Reemtsma.

Figure 7: Tom Starzl (born 1926). Image courtesy of Thomas E. Starzl.
 The use of non-human primates as kidney donors gained traction, notably by surgeons like Tom Starzl (Figure 7), who utilized baboons as donors in Colorado [18]. While achieving results similar to Reemtsma’s, long-term survivors were not attained. Others in the U.S. and France also had limited experiences [3].
The use of non-human primates as kidney donors gained traction, notably by surgeons like Tom Starzl (Figure 7), who utilized baboons as donors in Colorado [18]. While achieving results similar to Reemtsma’s, long-term survivors were not attained. Others in the U.S. and France also had limited experiences [3].
The First Heart Xenotransplant

James Hardy (Figure 8), renowned for the first human lung allotransplant in 1963, observed the positive health outcomes of some patients with chimpanzee kidney transplants. In 1964, Hardy aimed to conduct the initial clinical heart transplantation and acquired chimpanzees as potential donors, given the difficulty in finding a suitable deceased human donor [19]. Despite a less-than-ideal patient, ineligible for contemporary heart transplantation due to widespread vascular disease and a semi-comatose state, Hardy proceeded to transplant a chimpanzee heart as the patient was rapidly deteriorating. However, the chimpanzee’s heart could not sustain circulation and failed within hours [19,20].

Figure 8: James Hardy (1918–2003). Image courtesy of the late James Hardy.
Unlike the response to lung allotransplantation, public and medical reactions to heart xenotransplantation were unfavorable, deterring further attempts by Hardy and colleagues. Barnard and colleagues later accomplished cardiac allotransplantation in 1967 and conducted two cardiac xenotransplants [21].
Interestingly, the consent form for Hardy’s operation, signed by a close relative due to the patient’s semi-comatose state, did not mention the potential use of an animal heart, reflecting the legal standards of that time, where this “informed” consent was considered acceptable.
Liver Xenotransplantation
Tom Starzl (Figure 7), a prominent figure in kidney and liver allotransplantation, conducted several liver transplants between non-human primates and young patients in Colorado during the 1960s, yielding limited success [13–21]. In the 1990s, after improving the immunosuppressive arsenal by adding tacrolimus, Starzl and his team in Pittsburgh executed two liver transplants from baboons into adult patients, achieving survival of 70 days in one case [17]. Despite these attempts, the outcomes were not compelling enough to justify the continuation of this experimental clinical trial.
In the initial phases of clinical organ xenotransplantation, non-human primate species were primarily utilized as organ sources, with a few attempts involving pigs [18] and other non-primate mammals, albeit without significant success [3].
Xenotransplantation Using Pigs as Organ and Cell Sources
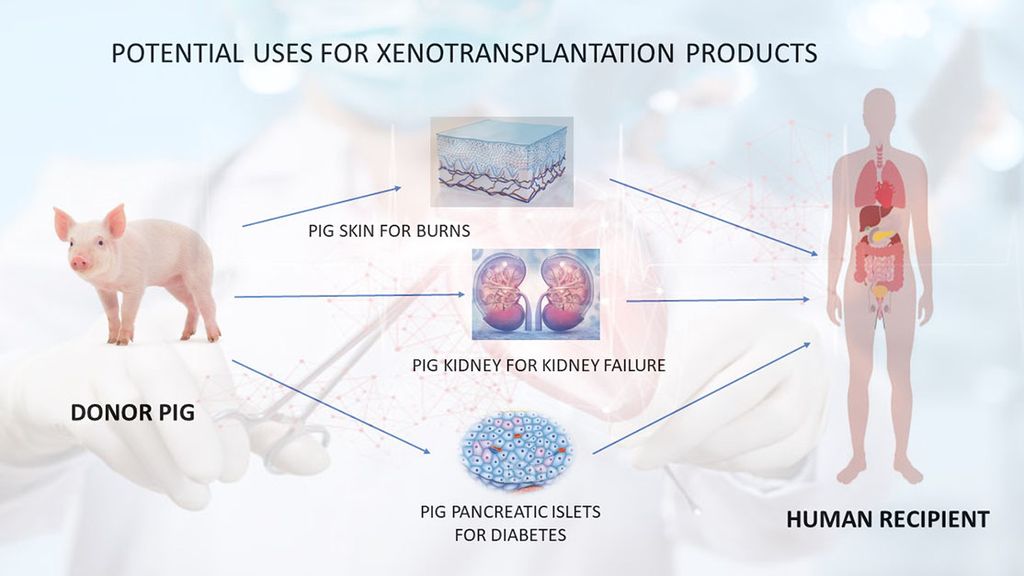
- Unlimited Supply: Pigs in xenotransplantation address the severe shortage of human organs, providing a limitless supply of donor organs.
- Timely Availability: This approach ensures the prompt availability of organs, eliminating prolonged patient waiting times and allowing immediate transplantation upon irreversible organ failure.
- Avoidance of Brain Death Effects: Xenotransplantation avoids adverse effects of brain death on donor organs, particularly the heart. Organs from healthy pigs excised under anesthesia bypass these issues.
- Reduced Microorganism Transfer Risk: Given controlled conditions and regular monitoring of the pig herd, pig organs minimize the risk of transferring novel microorganisms. This enhances the likelihood of the donor pig being free of known pathogenic organisms compared to deceased human donors.
- Cultural Barriers: Xenotransplantation can overcome cultural barriers to deceased organ donation in certain countries, significantly increasing transplant numbers.
- Risk Mitigation: This approach may mitigate risks associated with living donor liver transplants, which have led to donor deaths.
- Persistent Challenges: Significant immunological and pathophysiological challenges persist in pig xenotransplantation, reflecting the evolutionary divergence of pigs and humans.
- Evolutionary Hurdles: Overcoming these challenges, as described by Claus Hammer, involves “outwitting evolution.”
Table 1: The advantages and disadvantages of the pig vs baboon as a potential source of organs and cells for humans
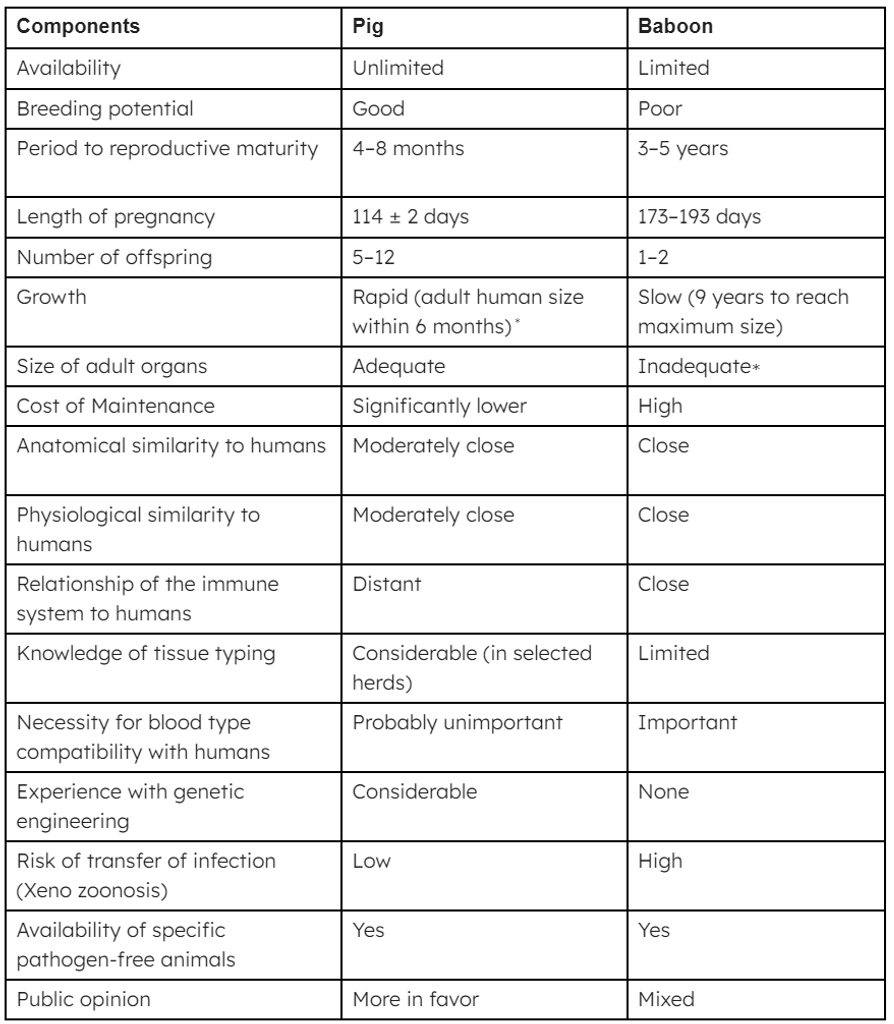 ∗Breeds of miniature swine are approximately 50% of the weight of domestic pigs at birth and sexual maturity and reach a maximum weight of roughly 30% of standard breeds. At full size, miniature swine are easier to house and to handle. Furthermore, inbred herds are available, though cloning of any pig can result in inbred herds if needed. Although MHC-identical miniature swine may have some specific immunologic advantage, the disadvantage is that they cannot be cross-bred with other pig strains in which a genetic modification has been introduced; if cross-breeding is carried out, MHC identity is lost.
∗Breeds of miniature swine are approximately 50% of the weight of domestic pigs at birth and sexual maturity and reach a maximum weight of roughly 30% of standard breeds. At full size, miniature swine are easier to house and to handle. Furthermore, inbred herds are available, though cloning of any pig can result in inbred herds if needed. Although MHC-identical miniature swine may have some specific immunologic advantage, the disadvantage is that they cannot be cross-bred with other pig strains in which a genetic modification has been introduced; if cross-breeding is carried out, MHC identity is lost.
Safety and Regulatory Aspects of Xenotransplantation
National regulatory concerns focus on the safety of pig organs or cell xenotransplantation, particularly regarding the transfer of porcine microorganisms to recipients and potentially the wider population[14]. To address this, stringent measures will house pigs in controlled environments and regularly screen them for potential pathogens. Consequently, organs and cells from these pigs are expected to be safer in this respect compared to allografts from recently brain-dead humans, where inadequate time is available for monitoring all potential infectious agents [14-16].
The primary concern in xenotransplantation revolves around endogenous retroviruses in every porcine cell’s genome. Despite initial worries, these viruses are generally believed to be weak and unlikely to pose significant problems, even in immunosuppressed recipients. No documented cases exist of these viruses transferring to humans or non-human primates exposed to pig tissues. Regulatory authorities will mandate strict monitoring for infectious complications in recipients and require the archiving of tissues from the source pig for an extended period [15].
Predicting Future Progress
In 1969, Sir Peter Medawar, a Nobel Prize-winning British scientist recognized as the father of transplant immunology, expressed optimism about solving the organ transplantation problem using xenografts within 15 years, a prediction that proved incorrect. In contrast, in 1995, Sir Roy Calne, another organ transplantation pioneer, anticipated xenotransplantation being “just around the corner,” albeit a very long one, a prediction that has been validated. Medawar and Calne were hopeful about the development of xenotransplantation. In contrast, Norman Shumway, the pioneer of heart transplantation, offered a more pessimistic view, stating that “xenotransplantation is the future of transplantation, and always will be.”
References
- Cooper DKC, Lanza RP. Xeno—The Promise of Transplanting Animal Organs into Humans. New York: Oxford University Press, 2000:1–274.
- Ekser B, Ezzelarab M, Hara H, van der Windt DJ, Wijkstrom M, Bottino R, Trucco M, Cooper DKC. Clinical xenotransplantation—the next great medical revolution? Lancet, in press.
- Taniguchi S, Cooper DKC. Clinical xenotransplantation: past, present and future. Ann R Coll Surg Engl 1997;79(1):13–19.
- Deschamps JY, Roux FA, Saï P, Gouin E. History of xenotransplantation. Xenotransplantation 2005;12(2):91–109.
- Reemtsma K. Xenotransplantation—a brief history of clinical experience: 1900–1965. Cooper DKC, Kemp E, Reemtsma K, White DJG, eds. Xenotransplantation: The Transplantation of Organs and Tissues Between Species (1st ed.). Heidelberg: Springer, 1991:9–22.
- Roux FA, Saï P, Deschamps JY. Xenotransfusions, past and present. Xenotransplantation 2007;14(3):208–216.
- Cooper DKC, Hara H, Yazer M. Genetically engineered pigs as a source for clinical red blood cell transfusion. Clin Lab Med 2010;30(2): 365–380.
- Gibson T. Zoografting: a curious chapter in the history of plastic surgery. Br J Plast Surg 1955;8(3):234–242.
- Cooper DKC. Xenografting: the early, early years. Xeno 1997; 5:21–22.
- Hara H, Cooper DKC. The immunology of corneal xenotransplantation: a review of the literature. Xenotransplantation 2010;17(5):338–349.
- Hara H, Cooper DKC. Xenotransplantation–the future of corneal transplantation? Cornea 2011;30(4):371–378.
- Hamilton D. The Monkey Gland Affair. London: Chatto and Windus, 1986.
- Real J. Voronoff [French]. Paris: Editors Stock, 2001.
- Matevossian E, Kern H, Hüser N, Doll D, Snopok Y, Nährig J, Altomonte J, Sinicina I, Friess H, Th orban S. Surgeon Yurii Voronoy (1895–1961)—a pioneer in the history of clinical transplantation: in memoriam at the 75th anniversary of the first human kidney transplantation. Transpl Int 2009;22(12):1132–1139.
- Lee RA. The Bizarre Careers of John R. Brinkley. Lexington, KY: University Press of Kentucky, 2002.
- Sgroi A, Bühler LH, Morel P, Sykes M, Noel L. International human xenotransplantation inventory. Transplantation 2010;90(6):597–603
- Reemtsma K, McCracken BH, Schlegel JU, Pearl MA, Pearce CW, DeWitt CV, Smith PE, Hewitt RL, Flinner RL, Creech O Jr. Renal heterotransplantation in man. Ann Surg 1964; 160:384–410.
- Starzl TE, Marchioro TL, Peters GN, Kirkpatrick CH, Wilson WE, Porter KA, Rifkind D, Ogden DA, Hitchcock CR, Waddell WR. Renal heterotransplantation from baboon to man: experience with 6 cases. Transplantation 1964; 2:752–776.
- Hardy JD, Kurrus FD, Chavez CM, Neely WA, Eraslan S, Turner MD, Fabian LW, Labecki TD. Heart transplantation in man: developmental studies and a case report. JAMA 1964; 188:1132–1140.
- Barnard CN. The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J 1967;41(48):1271–1274.
- Barnard CN, Wolpowitz A, Losman JG. Heterotopic cardiac transplantation with a xenograft for the assistance of the left heart in cardiogenic shock after cardiopulmonary bypass. S Afr Med J 1977;52(26):1035–1038.
- Bailey LL, Nehlsen-Cannarella SL, Concepcion W, Jolley WB. Baboon-to-human cardiac xenotransplantation in a neonate. JAMA 1985;254(23):3321–3329
About Docquity
If you need more confidence and insights to boost careers in healthcare, expanding the network to other healthcare professionals to practice peer-to-peer learning might be the answer. One way to do it is by joining a social platform for healthcare professionals, such as Docquity.
Docquity is an AI-based state-of-the-art private & secure continual learning network of verified doctors, bringing you real-time knowledge from thousands of doctors worldwide. Today, Docquity has over 400,000 doctors spread across six countries in Asia. Meet experts and trusted peers across Asia where you can safely discuss clinical cases, get up-to-date insights from webinars and research journals, and earn CME/CPD credits through certified courses from Docquity Academy. All with the ease of a mobile app available on Android & iOS platforms!







