Anthrax, a potentially deadly disease, demands swift and appropriate intervention. CDC guidelines underscore early diagnosis and treatment for improved survival. For nonpregnant adults, post-exposure prophylaxis (PEP) involves oral antimicrobial drugs over antitoxins, with a recommended 60-day course after aerosol exposure. Systemic anthrax treatment requires bactericidal agents, favoring combinations for toxin reduction. Pregnant and lactating individuals should follow specific regimens, considering safety. Children and neonates mirror adult guidelines with adjusted antimicrobial drug choices. Special considerations address anthrax complications, including meningitis and inhalation or ingestion exposure.

Recommendations for Anthrax Prevention and Treatment
► Nonpregnant Adults Aged ≥18 Years
Recommendation 1: PEP and Treatment Regimens for Cutaneous Anthrax Without Signs and Symptoms of Meningitis
- Choose a single antimicrobial drug.
- Continue or switch based on susceptibility testing.
- Use “PCN-S only” after determining penicillin susceptibility.
PEPAbx should be continued for 60 days after aerosol exposure. For nonaerosol exposures, continue PEPAbx for 7 days. For cutaneous Anthrax without meningitis, treat for 7-10 days.
Recommendation 2: Treatment Regimen for Systemic Anthrax With or Without Meningitis
- Choose two bactericidal drugs from different classes.
- Continue or switch based on susceptibility testing.
- Use “PCN-S only” after determining penicillin susceptibility.
- Choose a single antitoxin as adjunctive therapy.
Consider alternative regimens based on contraindications or tolerability. Minocycline and Doxycycline are preferred.
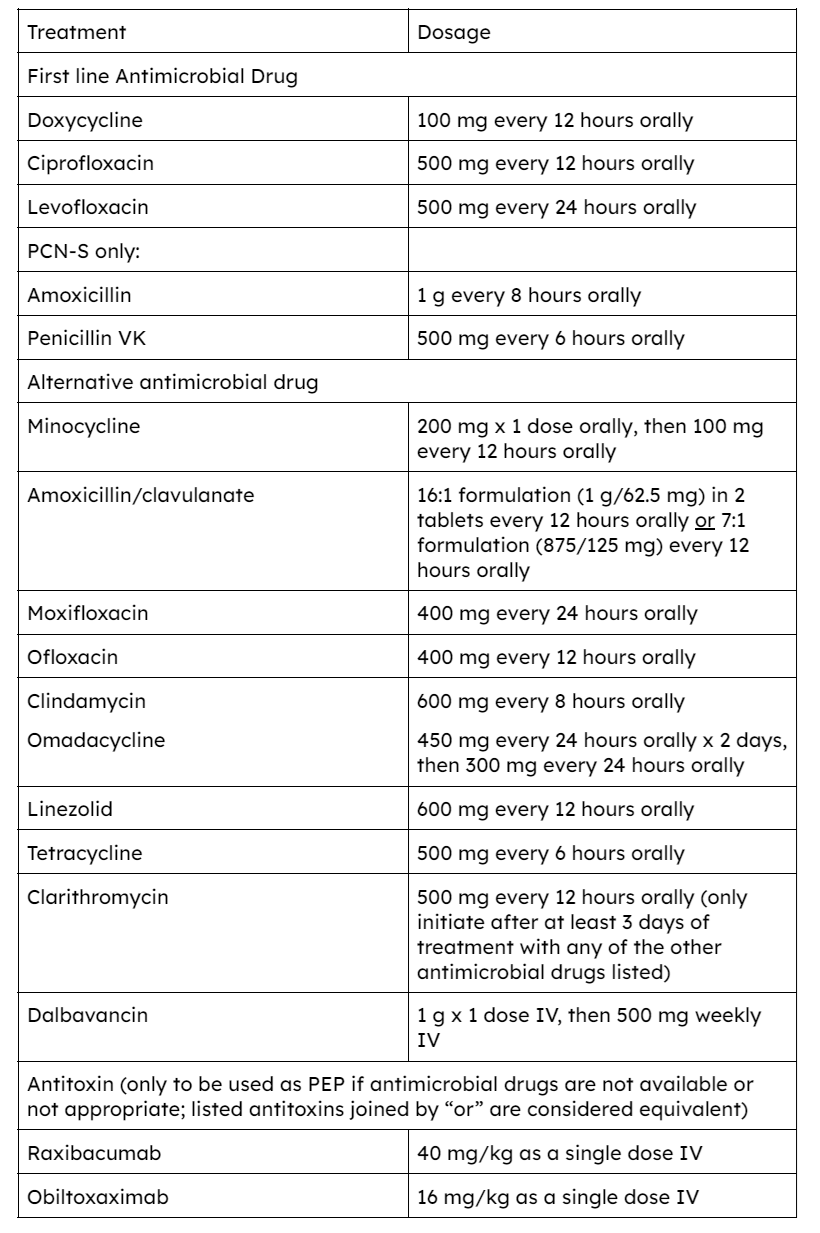
Table 1: PEP for nonpregnant adults aged ≥18 years after exposure to B. anthracis
► Pregnant and Lactating Persons
Recommendation 3: PEP and Treatment Regimens for Cutaneous Anthrax Without Signs and Symptoms of Meningitis
- Choose a single antimicrobial drug.
- Evaluate fluoroquinolones cautiously, avoiding tetracycline.
- Continue or switch based on susceptibility testing.
- Use “PCN-S only” after determining penicillin susceptibility.
- Choose a single antitoxin if no antimicrobial drugs are available.
PEPAbx should be taken for 60 days for aerosol exposures. For nonaerosol exposures, take PEPAbx for 7 days. Treat cutaneous Anthrax for 7-10 days.
Recommendation 4: Treatment Regimens for Systemic Anthrax With or Without Meningitis
- Choose two bactericidal drugs from different classes.
- Continue or switch based on susceptibility testing.
- Use “PCN-S only” after determining penicillin susceptibility.
- Choose a single antitoxin as adjunctive therapy.
Minocycline and Doxycycline are preferred. Treatment duration should be 2 weeks or longer, lasting 60 days.

Table 2: PEP for pregnant or lactating persons, aged >18 years after exposure to B. anthracis
► Children (≥1 Month to <18 Years)
Recommendation 5: PEP and Treatment Regimens for Cutaneous Anthrax Without Signs and Symptoms of Meningitis
- Choose a single antimicrobial drug.
- Continue or switch based on susceptibility testing.
- Use “PCN-S only” after determining penicillin susceptibility.
- Prefer penicillin-class antimicrobial for susceptible strains.
PEPAbx should be continued for 60 days for aerosol exposure. For nonaerosol exposures, continue for 7 days. Treat cutaneous Anthrax for 7-10 days.
Recommendation 6: Treatment Regimens for Systemic Anthrax With or Without Meningitis
- Choose two bactericidal drugs from different classes.
- Continue or switch based on susceptibility testing.
- Use “PCN-S only” after determining penicillin susceptibility.
- Choose a single antitoxin as adjunctive therapy.
Minocycline and Doxycycline are preferred. Treatment duration should be 2 weeks or longer, lasting 60 days.

Table 3: PEP for children aged ≥1 month to <18 years after exposure to B. anthracis
► Preterm and Full-Term Newborns
Recommendation 7: PEP and Treatment Regimens for Cutaneous Anthrax Without Signs and Symptoms of Meningitis
- Choose a single antimicrobial drug.
- Continue or switch based on susceptibility testing.
- Use “PCN-S only” after determining penicillin susceptibility.
- Choose a single antitoxin if no antimicrobial drugs are available.
PEPAbx should be continued for 60 days after aerosol exposure. For cutaneous Anthrax without aerosol exposure, PEPAbx is not required.
Recommendation 8: Treatment Regimens for Systemic Anthrax With or Without Meningitis
- Choose two bactericidal drugs from different classes.
- Continue or switch based on susceptibility testing.
- Use “PCN-S only” after determining penicillin susceptibility.
- Choose a single antitoxin as adjunctive therapy.
Minocycline and Doxycycline are preferred. Treatment duration should be 2 weeks or longer, lasting 60 days.

Table 4: PEP for preterm and full-term neonates 32-44 weeks postmenstrual age (gestational age plus chronologic age) after exposure to B. anthracis
► Special Considerations for Inhalation and Ingestion Anthrax
Recommendation 9: Special Considerations for Anthrax Meningitis
- Drain pleural effusion early and aggressively.
- Isolate potentially contaminated patients until decontamination.
- Evaluate all patients with systemic symptoms of meningitis.
- Use a screening tool when conventional diagnostics are limited.
Adjunctive therapy for meningitis includes mannitol or hypertonic saline for cerebral edema. Consider steroids if clinically indicated, but their survival benefit is not demonstrated.
Conclusion
CDC’s comprehensive guidelines outline crucial protocols for preventing and treating Anthrax, acknowledging its bioweapon potential. These recommendations, subject to updates with evolving research, emphasize tailored approaches for diverse populations. Early intervention, individualized drug regimens, and addressing complications like meningitis are pivotal. Though a persistent concern, Anthrax can be effectively managed through adherence to these guidelines, ensuring a robust response to both natural occurrences and potential bioterrorism events. Continued research and technological advancements will further refine these guidelines for enhanced preparedness.
Reference
Morbidity and Mortality Weekly Report CDC Guidelines for the Prevention and Treatment of Anthrax, 2023 [Internet]. 2023 [cited 2023 Nov 23]. Available from: https://www.cdc.gov/mmwr/volumes/72/rr/pdfs/rr7206a1-H.pdf







